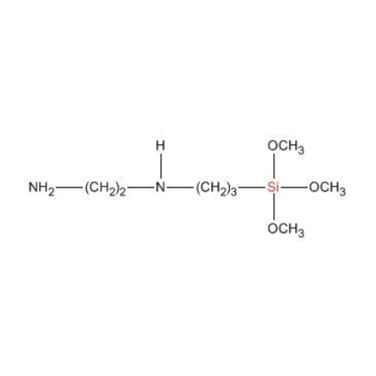
N-[3-(Trimethoxysilyl)propyl]ethylenediamine
Aminoethyl aminopropyl trimethoxysilane is a kind of diaminosilane, commonly known as KH-792. Its molecular structure contains two amino functional groups (a primary ammonia and a secondary ammonia) and three hydrolyzed alkoxy - methoxy group. This dual reactivity enables it to improve the degree of binding, bonding and compatibility between inorganic materials (glass, metal, filler) and organic polymers (thermosetting resins, plastics, elastomers) through two-way chemical reactions. Then the mechanical properties of resin matrix composites or the bonding strength and water resistance of resin coatings can be improved. In different applications, it can be used as coupling agent, adhesion enhancer, curing agent, surface modifier of fillers, etc.
KH-792 is trimethoxysilane, and its hydrolysis rate is significantly faster than triethoxysilane SCA-A20E, which can provide faster reaction and curing speed. However, the by-reactant of its hydrolysis reaction is methanol, which is less environmentally friendly.
Efficacy and use:
1. as an additive or prepared into the bottom coating liquid and used in phenolic aldehyde, urea aldehyde, furan, polyurethane, silicone, epoxy, nitrile, phenolic, acrylic and other coatings, ink, adhesives and sealants, to improve the adhesion of resin coating, corrosion resistance, weather resistance, boiling resistance and scrub resistance, prolong the service life, and improve the dispersion and bonding of the makeup, filler in the resin phase.
2. Resin sand casting and resin abrasives are used to improve the binding force and water resistance of resin and silica sand or abrasive.
3. mineral filler or glass fiber filled plastic, rubber, resin and low smoke halogen-free flame retardant cable material to improve the dispersion and combination of filler and fiber in the resin phase.
4. inorganic mineral filler, flame retardant and glass fiber surface treatment, to improve its dispersibility, compatibility, binding force and enhance the effect in the resin phase.
Storage:
Store indoors away from light, keep ventilated, cool and dry. Make sure the lid is well sealed and effectively keeps out the air. Moisture in the air will react with it, causing it to hydrolyze, polymerize and deteriorate.